Editing in Cells
Overview
One area of research in the Doudna is using CRISPR-Cas systems as tools to edit genes in plants and mammals. Together, we are establishing genetic-based strategies to better combat human diseases and to engineer disease-resistant staple crops. Additionally, we are advancing the safe and effective delivery of CRISPR-Cas tools into cells targeted for genome engineering.
Editing in disease: CRISPR-Cas gene editing for the study and treatment of human diseases
CRISPR-Cas is a gene-editing technology that has the potential to dramatically reshape the treatment of genetic diseases by replacing or deleting problematic genes. Cas proteins can be delivered non-genetically in the form of a ribonucleoprotein (RNP) complex – one method for direct and efficient gene editing in cells – our laboratory has recently developed Cas9 RNPs that are cell penetrant in the context of the adult mouse brain. We are currently exploring therapeutic use of Cas9 as a method to treat Huntington’s Disease (HD). HD is a neurodegenerative disorder caused by a genetically dominant, CAG trinucleotide expansion in the Huntingtin (HTT) gene, and suppression of mutant Huntington protein (mHTT) is currently being explored as a therapeutic method to treat HD. We are using Cas9 RNP technology to reduce the levels of mHTT, with the goal of halting the primary disease mechanism and progression of HD.
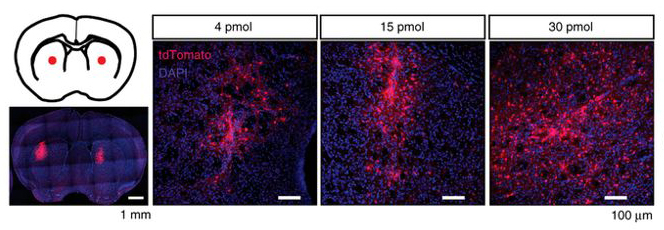 Along with Huntington’s disease, we are also interested in using CRISPR-based technologies to study and treat various other brain diseases. In particular, we are focusing our efforts on primary glioblastoma (GBM), which is both the most common and most devastating form of malignant brain cancer in adults. Current treatment options are limited, with GBM progression leading to death within 2 years of diagnosis in 90% of cases. In collaboration with the Costello lab at UCSF and the Fellmann lab at the Gladstone Institutes, we are using a variety of CRISPR strategies including Cas9 circular permutants and cell-penetrating Cas9 ribonucleoproteins to understand and exploit genetic vulnerabilities of GBM.
Along with Huntington’s disease, we are also interested in using CRISPR-based technologies to study and treat various other brain diseases. In particular, we are focusing our efforts on primary glioblastoma (GBM), which is both the most common and most devastating form of malignant brain cancer in adults. Current treatment options are limited, with GBM progression leading to death within 2 years of diagnosis in 90% of cases. In collaboration with the Costello lab at UCSF and the Fellmann lab at the Gladstone Institutes, we are using a variety of CRISPR strategies including Cas9 circular permutants and cell-penetrating Cas9 ribonucleoproteins to understand and exploit genetic vulnerabilities of GBM.
Editing in plants: CRISPR-Cas gene editing for the improvement of staple crops
Traditional genetic manipulation and trait selection of food staple crops is a long and cumbersome process. In collaboration with Brian Staskawicz’s UC Berkeley laboratory, we are working on advancing agriculturally relevant CRISPR/Cas9-mediated gene editing.
We are particularly interested in developing homology-directed repair (HDR) tools in plant crop species in order to exploit genome engineering beyond producing NHEJ-mediated indel knockouts. Additionally, we are establishing novel delivery methods of CRISPR-Cas9 reagents to overcome the physical barrier imposed by the plant cell wall and thus improve gene editing efficiency and scalability in a transgene-free manner. We hope that these efforts will improve the disease resistance and yield of crops, with particular emphasis on the improvement of tomatoes, rice, and wheat.
Delivery of CRISPR-Cas tools to disease-relevant human cells
The therapeutic use of CRISPR-Cas requires the effective transportation of gene editing tools into disease-relevant cells and tissues. We therefore are developing strategies for the effective delivery of gene editing tools for both ex vivo and in vivo use in mammals. Recently, our laboratory has generated cell-penetrating Cas9 ribonucleoproteins by the iterative addition of nuclear-localization signals:
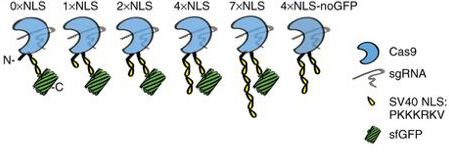 These cell-penetrating Cas9 ribonucleoproteins are effective at mediating gene editing in the neuronal cells of mice and are a new strategy for directly altering disease-causing genes in the brain. We collaborate closely with the UC Berkeley laboratories of Matt Francis and Ross Wilson to design and test both viral and non-viral strategies for the delivery of CRISPR-Cas tools. We are building upon our initial successes to design new strategies for the effective delivery of Cas9 ribonucleoproteins.
These cell-penetrating Cas9 ribonucleoproteins are effective at mediating gene editing in the neuronal cells of mice and are a new strategy for directly altering disease-causing genes in the brain. We collaborate closely with the UC Berkeley laboratories of Matt Francis and Ross Wilson to design and test both viral and non-viral strategies for the delivery of CRISPR-Cas tools. We are building upon our initial successes to design new strategies for the effective delivery of Cas9 ribonucleoproteins.



